Triangulene
 | |
| Names | |
|---|---|
| Preferred IUPAC name Dibenzo[cd,mn]pyrene-4,8-diyl | |
| Other names [3]Triangulene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C22H12 |
| Molar mass | 276.338 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
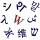

Triangulene (also known as Clar's hydrocarbon) is the smallest triplet-ground-state polybenzenoid.[1] It exists as a biradical with the chemical formula C
22H
12.[2] It was first hypothesized by Czech chemist Erich Clar in 1953.[3] Its first confirmed synthesis was published in a February 2017 issue of Nature Nanotechnology, in a project led by researchers David Fox and Anish Mistry at the University of Warwick in collaboration with IBM.[4] Other attempts by Japanese researchers have been successful only in making substituted triangulene derivatives.[5]
A six-step synthesis yielded two isomers of dihydrotriangulene which were then deposited on xenon or copper base. The researchers used a combined scanning tunneling and atomic force microscope (STM/AFM) to remove individual hydrogen atoms. The synthesized molecule of triangulene remained stable at high-vacuum low-temperature conditions for four days, giving the scientists plenty of time to characterize it (also using STM/AFM).[4]
[n]Triangulenes
Triangulene, as defined here, is a member of a wider class of [n]triangulenes, where n is the number of hexagons along an edge of the molecule. Thus, triangulene may also be referred to as [3]triangulene.
Theory
A tight-binding description of the molecular orbitals of [n]triangulenes predicts[6] that [n]triangulenes have (n − 1) unpaired electrons, which are associated to (n − 1) non-bonding states. When electron–electron interactions are included, theory predicts[6][7][8] that the ground state total spin quantum number S of [n]triangulenes is S = n − 1/2. Thus, [3]triangulenes are predicted to have an S = 1 ground state. The intramolecular exchange interaction in triangulene, which determines the energy difference between the S = 1 ground state and the S = 0 excited state, is predicted to be the largest[9] among all polycyclic aromatic hydrocarbon (PAH) diradicals, due to maximum overlap of the wave function of the unpaired electrons.
The ground state spin of [n]triangulenes can be rationalized[6] in terms of a theorem[10] by Elliot H. Lieb, which relates, for a bipartite lattice, the ground state spin of the Hubbard model at half filling to the sublattice imbalance.
Experiments
So far, the ultra-high vacuum on-surface syntheses of [n]triangulenes with n = 3,[4] 4,[11] 5[12] and 7[13] (the hitherto largest triangulene homologue) have been reported. In addition, the on-surface synthesis of [3]triangulene dimers[14] has also been reported, where inelastic electron tunneling spectroscopy provides a direct evidence of a strong antiferromagnetic coupling between the triangulenes. In 2021, an international team of researchers reported the fabrication of [3]triangulene-based quantum spin chains on a gold surface,[15] where signatures of both spin fractionalization and Haldane gap were observed.
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "biradical". doi:10.1351/goldbook.B00671
- ^ "triangulene | C22H12 | ChemSpider". www.chemspider.com. Retrieved 2017-02-19.
- ^ Ball, Philip (February 2017). "Elusive triangulene created by moving atoms one at a time". Nature. 542 (7641): 284–285. Bibcode:2017Natur.542..284B. doi:10.1038/nature.2017.21462. PMID 28202993. S2CID 4398214.
- ^ a b c Pavliček, Niko; Mistry, Anish; Majzik, Zsolt; Moll, Nikolaj; Meyer, Gerhard; Fox, David J.; Gross, Leo (April 2017). "Synthesis and characterization of triangulene" (PDF). Nature Nanotechnology. 12 (4): 308–311. Bibcode:2017NatNa..12..308P. doi:10.1038/nnano.2016.305. PMID 28192389.
- ^ Morita, Yasushi; Suzuki, Shuichi; Sato, Kazunobu; Takui, Takeji (2011). "Synthetic organic spin chemistry for structurally well-defined open-shell graphene fragments". Nature Chemistry. 3 (3): 197–204. Bibcode:2011NatCh...3..197M. doi:10.1038/nchem.985. PMID 21336324.
- ^ a b c Fernández-Rossier, J.; Palacios, J. J. (23 October 2007). "Magnetism in Graphene Nanoislands". Physical Review Letters. 99 (17): 177204. arXiv:0707.2964. Bibcode:2007PhRvL..99q7204F. doi:10.1103/PhysRevLett.99.177204. hdl:10045/25254. PMID 17995364. S2CID 9697828.
- ^ Wang, Wei L.; Meng, Sheng; Kaxiras, Efthimios (1 January 2008). "Graphene NanoFlakes with Large Spin". Nano Letters. 8 (1): 241–245. Bibcode:2008NanoL...8..241W. doi:10.1021/nl072548a. PMID 18052302.
- ^ Güçlü, A. D.; Potasz, P.; Voznyy, O.; Korkusinski, M.; Hawrylak, P. (10 December 2009). "Magnetism and Correlations in Fractionally Filled Degenerate Shells of Graphene Quantum Dots". Physical Review Letters. 103 (24): 246805. arXiv:0907.5431. Bibcode:2009PhRvL.103x6805G. doi:10.1103/PhysRevLett.103.246805. PMID 20366221. S2CID 18754119.
- ^ Ortiz, Ricardo; Boto, Roberto A.; García-Martínez, Noel; Sancho-García, Juan C.; Melle-Franco, Manuel; Fernández-Rossier, Joaquı́n (11 September 2019). "Exchange Rules for Diradical π-Conjugated Hydrocarbons". Nano Letters. 19 (9): 5991–5997. arXiv:1906.08544. Bibcode:2019NanoL..19.5991O. doi:10.1021/acs.nanolett.9b01773. PMID 31365266. S2CID 195218794.
- ^ Lieb, Elliott H. (6 March 1989). "Two theorems on the Hubbard model". Physical Review Letters. 62 (10): 1201–1204. Bibcode:1989PhRvL..62.1201L. doi:10.1103/PhysRevLett.62.1201. PMID 10039602.
- ^ Mishra, Shantanu; Beyer, Doreen; Eimre, Kristjan; Liu, Junzhi; Berger, Reinhard; Gröning, Oliver; Pignedoli, Carlo A.; Müllen, Klaus; Fasel, Roman; Feng, Xinliang; Ruffieux, Pascal (10 July 2019). "Synthesis and Characterization of π-Extended Triangulene" (PDF). Journal of the American Chemical Society. 141 (27): 10621–10625. doi:10.1021/jacs.9b05319. PMID 31241927. S2CID 195696890.
- ^ Su, Jie; Telychko, Mykola; Hu, Pan; Macam, Gennevieve; Mutombo, Pingo; Zhang, Hejian; Bao, Yang; Cheng, Fang; Huang, Zhi-Quan; Qiu, Zhizhan; Tan, Sherman J. R.; Lin, Hsin; Jelínek, Pavel; Chuang, Feng-Chuan; Wu, Jishan; Lu, Jiong (July 2019). "Atomically precise bottom-up synthesis of π-extended [5]triangulene". Science Advances. 5 (7): eaav7717. Bibcode:2019SciA....5.7717S. doi:10.1126/sciadv.aav7717. PMC 6660211. PMID 31360763.
- ^ Mishra, Shantanu; Xu, Kun; Eimre, Kristjan; Komber, Hartmut; Ma, Ji; Pignedoli, Carlo A.; Fasel, Roman; Feng, Xinliang; Ruffieux, Pascal (2021). "Synthesis and characterization of [7]triangulene". Nanoscale. 13 (3): 1624–1628. doi:10.1039/d0nr08181g. PMID 33443270. S2CID 231605335.
- ^ Mishra, Shantanu; Beyer, Doreen; Eimre, Kristjan; Ortiz, Ricardo; Fernández-Rossier, Joaquín; Berger, Reinhard; Gröning, Oliver; Pignedoli, Carlo A.; Fasel, Roman; Feng, Xinliang; Ruffieux, Pascal (13 July 2020). "Collective All-Carbon Magnetism in Triangulene Dimers". Angewandte Chemie International Edition. 59 (29): 12041–12047. arXiv:2003.00753. doi:10.1002/anie.202002687. PMC 7383983. PMID 32301570.
- ^ Mishra, Shantanu; Catarina, Gonçalo; Wu, Fupeng; Ortiz, Ricardo; Jacob, David; Eimre, Kristjan; Ma, Ji; Pignedoli, Carlo A.; Feng, Xinliang; Ruffieux, Pascal; Fernández-Rossier, Joaquín; Fasel, Roman (13 October 2021). "Observation of fractional edge excitations in nanographene spin chains". Nature. 598 (7880): 287–292. arXiv:2105.09102. Bibcode:2021Natur.598..287M. doi:10.1038/s41586-021-03842-3. PMID 34645998. S2CID 234777902.
- v
- t
- e
- Benz[a]anthracene
- Benzo[a]fluorene
- Benzo[c]fluorene
- Benzo[c]phenanthrene
- Chrysene
- Fluoranthene
- Pyrene
- Tetracene
- Triphenylene
- Tricyclobutabenzene
- Benz[e]acephenanthrylene
- Benzopyrene
- Benzo[a]pyrene
- Benzo[e]pyrene
- 6H-Benzo[cd]pyrene(Olympicene)
- Benzo[a]fluoranthene
- Benzo[b]fluoranthene
- Benzo[j]fluoranthene
- Benzo[k]fluoranthene
- trans-Bicalicene
- Dibenz[a,h]anthracene
- Dibenz[a,j]anthracene
- Pentacene
- Perylene
- Picene
- Tetraphenylene
- Anthanthrene
- Benzo[ghi]perylene
- Corannulene
- Dibenzopyrenes
- Hexacene
- Triangulene
- Zethrene










