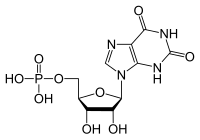
Ksantozin monofosfat
| Ksantozin monofosfat | |||
|---|---|---|---|
 | |||
| IUPAC ime |
| ||
| Drugi nazivi | ksantinski ribotid, XMP | ||
| Identifikacija | |||
| CAS registarski broj | 523-98-8  Y Y | ||
| PubChem[1][2] | 122280 | ||
| ChemSpider[3] | 66054  Y Y | ||
| KEGG[4] | C00655 | ||
| MeSH | Xanthosine+monophosphate | ||
| ChEBI | CHEBI:15652 | ||
| Jmol-3D slike | Slika 1 | ||
| |||
| |||
| Svojstva | |||
| Molekulska formula | C10H13N4O9P | ||
| Molarna masa | 364,206 g/mol | ||
| Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje (25 °C, 100 kPa) materijala | |||
| Infobox references | |||
Ksantozin monofosfat (XMP[5]) je intermedijer u purinskom metabolizmu.[6] On je ribonukleosidni monofosfat. XMP se formira iz inozin monofosfata posredstvom IMP dehidrogenaze, dok se guanozin monofosfat (GMP) formira posredstvom GMP sintaze.
Reference
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519. edit
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846. edit
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gogia, S.; Balaram, H.; Puranik, M. (May 2011). „Hypoxanthine guanine phosphoribosyltransferase distorts the purine ring of nucleotide substrates and perturbs the pKa of bound xanthosine monophosphate.”. Biochemistry 50 (19): 4184-93. DOI:10.1021/bi102039b. PMID 21486037.
- ↑ McMurry 2007: str. 1007
Literatura
- McMurry, John (2007). Organic chemistry: a biological approach. Cengage Learning. str. 1007-. ISBN 978-0-495-01525-3. Pristupljeno 26. 3. 2012.
- Sigel, H; Operschall, BP; Griesser, R (2009). „Xanthosine 5'-monophosphate (XMP). Acid-base and metal ion-binding properties of a chameleon-like nucleotide”. Chemical Society reviews 38 (8): 2465-94. DOI:10.1039/b902181g. PMID 19623361.
- Egli, M; Pallan, PS (2010). „Crystallographic studies of chemically modified nucleic acids: A backward glance”. Chemistry & Biodiversity 7 (1): 60-89. DOI:10.1002/cbdv.200900177. PMC 2905155. PMID 20087997.
Vidi još
Spoljašnje veze
 | Portal Hemija |
- p
- r
- u
| |||||







